Introduction
There is a substantial underrepresentation of ethnic minorities within oncology studies. Specifically, Black, Asian and Hispanic patients have a low participation rate in US based randomized clinical trials (RCT). Furthermore, patients living with HIV (PLWH) or chronic viral hepatitis are often systematically excluded from these studies. Diffuse large B-cell lymphoma (DLBCL) is one of the most common malignancies associated with HIV and viral hepatitis. Given these characteristics, it is imperative that studies reporting on safety and efficacy of new treatments mirror real-world populations.
Objective:
The primary objective of our study was to describe the demographic characteristics of patients who have participated in DLBCL phase II and III randomized US clinical trials; evaluating each group's enrollment fraction compared to US DLBCL prevalence. The secondary objective was analyzing the inclusion of PLWH and chronic viral hepatitis in US based DLBCL RCT.
Methods and Measures:
We queried PubMed, ClinicalTrials.gov, as well as ASCO and ASH meeting abstracts for phase II and III RCTs on DLBCL from 2000 to 2020, including chimeric antigen receptor T cells (CAR-T) therapy studies. Trials that did not report race and ethnicity or recruited outside of the US were excluded. We analyzed enrollment data and compared to DLBCL prevalence demographics obtained from the SEER 18 Registries. Enrollment fraction (EF) is defined as the number of trial enrollees divided by the estimated US prevalence of DLBCL.
Results:
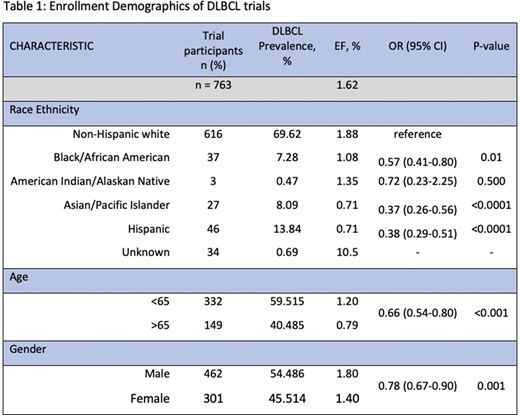
We identified 51 trials between 2000 and 2020 with only 25 (49%) studies reporting race and ethnicity. Only 9 trials enrolled solely in the US which comprised of 763 patients. Compared with an EF of 1.88% among Non-Hispanic whites, lower EF was noted in Blacks (1.08%; P=0.0009), Hispanics (0.71; P=<0.0001) and Asian patients (0.71%; P=<0.0001). The EF of patients < 65 years old was significantly higher than those aged 65 or more (1.2 vs 0.79%, P=<0.0001). The EF of males and females was 1.8 vs 1.4%, respectively (P=0.001). PLWH and/or chronic viral hepatitis were excluded in 89% and 67% of the clinical trials analyzed (Table 1).
Conclusions:
US based clinical trials for DLBCL have significant underrepresentation of Hispanic, Black and Asian patients. Moreover, despite being commonly associated with HIV and chronic viral hepatitis, patients with these comorbidities are typically excluded from clinical trials. Pharmacoethnicity, ethnic differences in susceptibility to the effects of therapy, remains a challenge in oncology because clinical trials have significant underrepresentation of minorities. Efforts are needed to address the ascertainment bias and improve external validity of RCTs in DLCBL. Reasons for clinical trial underrepresentation are complex and multifactorial including lack of access, cultural differences and healthcare system mistrust. Healthcare professionals must address the inequalities through awareness and education with the objective of increasing enrollment of minorities in clinical trials. Although there have been efforts to increase enrollment of patients with HIV and chronic viral hepatitis in clinical trials, it remains inadequate. In accordance to recent recommendations by the FDA, investigators should consider having a predefined plan for inclusion of such patients.
Sandoval-Sus:Massive Bio: Consultancy; Janssen: Consultancy; MorphoSys US: Consultancy; Celgene: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal